In addition to those described on the Group Members tab, we have a number of other very valuable and fruitful collaborations.
1. We work with Prof David Procter on understanding organic transformations catalysed by SmI2. This includes an alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres, and radical cyclisations of acyclic esters.
2. We have several ongoing projects with Centre for Radiochemistry Research co-Director, Prof Steve Liddle, focussing on the electronic structure and bonding in early actinide compounds. We have recently studied a highly unusual uranium(V)-dinitrogen complex, explored the structure-directing role of 5f orbital overlap-driven covalency, and reported a tri-thorium cluster featuring sigma aromatic Th-Th bonding:
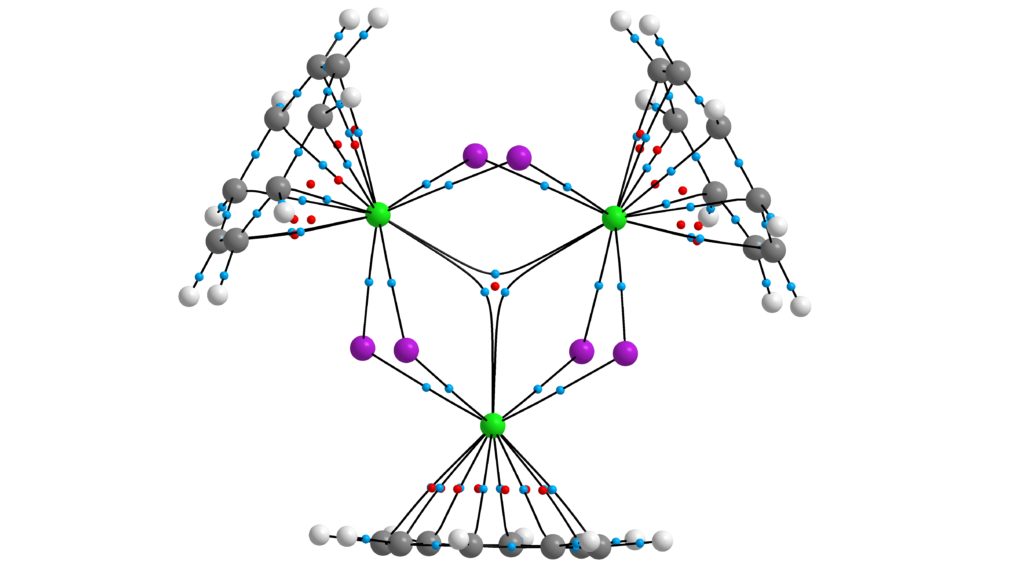 QTAIM molecular graph of [{Th(η8-C8H8)(μ3-Cl)2}3K2]
QTAIM molecular graph of [{Th(η8-C8H8)(μ3-Cl)2}3K2]
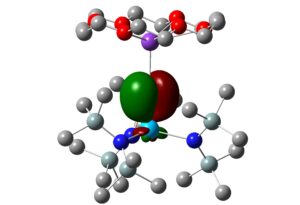
3. With Prof Polly Arnold at Berkeley, we study the organometallic chemistry of the 4f and 5f elements. For example, we have employed variable, high-pressure single-crystal X-ray crystallography, and density functional theory to characterise pressure induced uranium C-H agostic bonds (see image below) and pyramidalization in three-coordinate uranium tris(amido) and tris(arylthiolate) molecules, and have studied small molecule activation by uranium tris(aryloxides), and organometallic neptunium (III) complexes.
QTAIM molecular graph of [UN”2]2(μ-η6:η6-C6H6) (N” = N(SiMe3)2) at 3.2 GPa – note the bond paths between the uranium atoms and the hydrogens highlighted in yellow
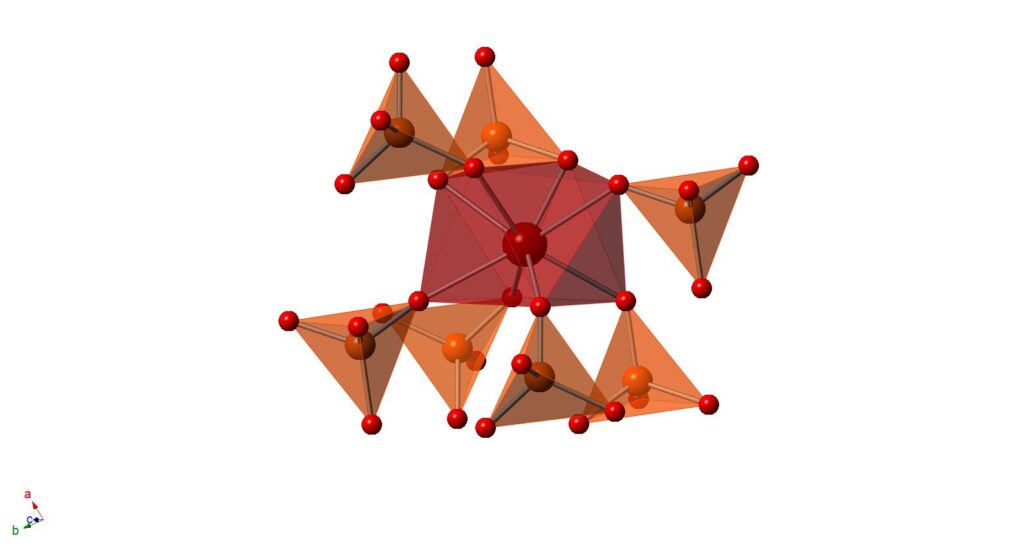
4. With Prof Tom Albrecht-Schoenzart at the Colorado School of Mines, we have studied f block chromates, and in particular the differences in the electronic structure between CsAm(CrO4)2 and its Sm and Eu analogues.
Polyhedral representation of CsAm(CrO4)2.
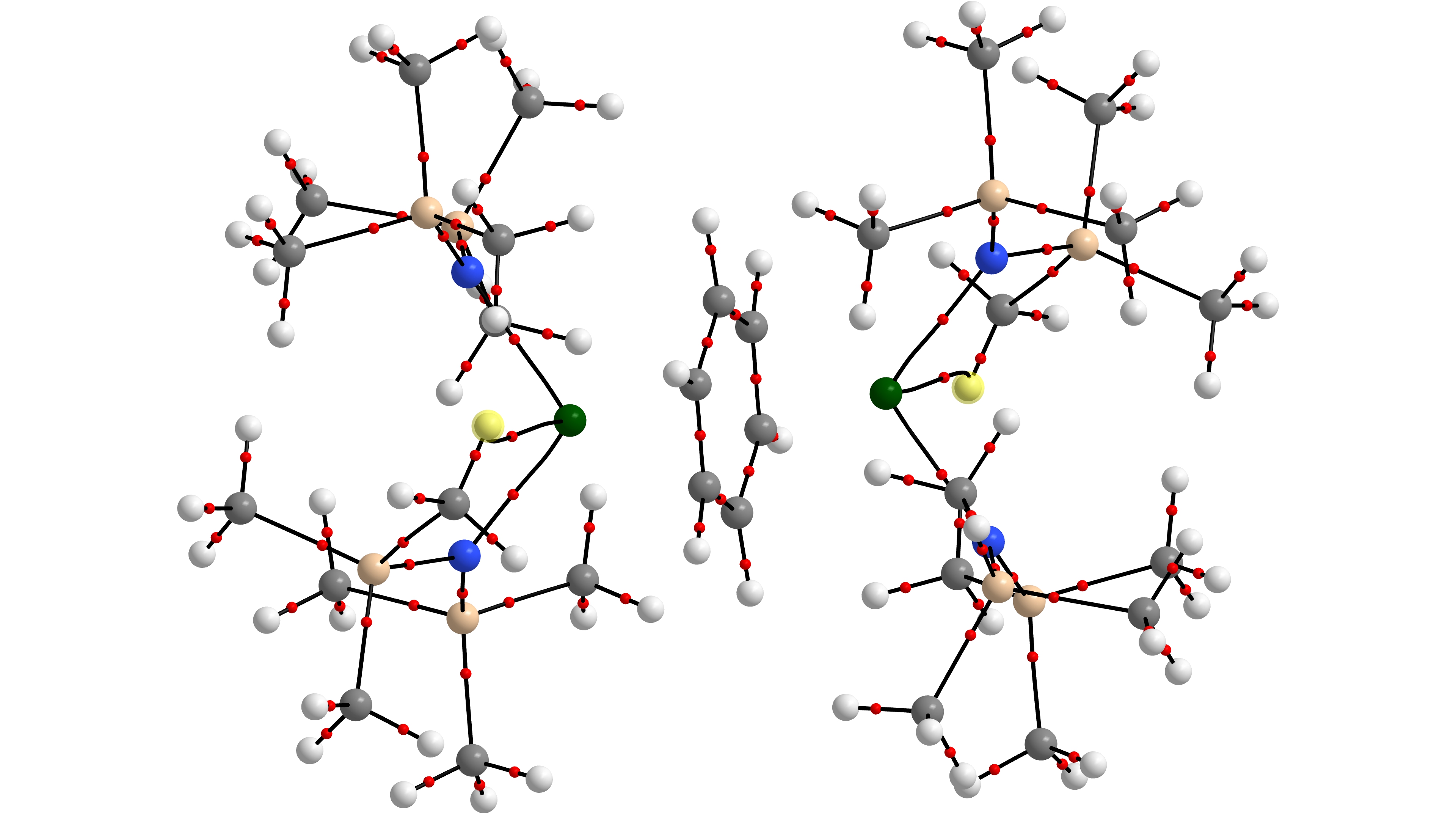
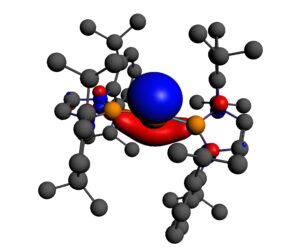
5. With Prof Trevor Hayton of the University of California at Santa Barbara, we have studied the organometallic chemistry of thorium and uranium. The An-E triple bonds (see below) in [K(18-crown-6)][ThE(NR2)3] (E = O, S; R = SiMe3) are found to be more ionic than their uranium analogues, with more 6d than 5f character. We have also studied chalcogenido-substituted analogues of the uranyl ion, [OUE]2+ (E = S, Se), Ce(IV) oxo chemistry, and a “masked” terminal zinc sulphide.
Th≡O Natural Localised Molecular Orbitals of [K(18-crown-6)][ThO(NR2)3] (H atoms not shown)
6. We have previously worked closely with Prof Philip Mountford and Prof Simon Aldridge at Oxford. Highlights include acyclic two-coordinate silylenes, thermally robust monomeric Ga(II), In(II) and Tl(II) systems and extensive studies of alkaline earth-transition metal bonding.
DFT-calculated (PBE) canonical SOMO of Ga[B(NDippCH)2)]2 (H atoms not shown)
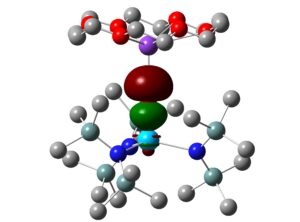
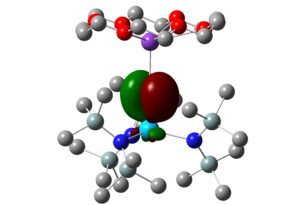
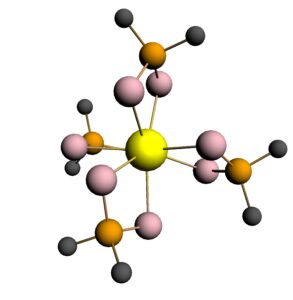
7. We have also worked with Dr Andrew Gaunt of the Los Alamos National Laboratory in the USA. Here, the focus has been on extending the organometallic and coordination chemistry of the actinides to the transuranic elements, especially plutonium, and we have probed the electronic structures of model An(IV) complexes with diselenophosphinate ligands, An(Se2PMe2)4 (An = Th-Pu; see below), using molecular orbital and QTAIM analyses.
Ball and stick image of Th(Se2PMe2)4 (H atoms not shown)